Easy DICOM Viewer can be used to anonymize DICOM tag values in your DICOM files. There are 3 options you can select:
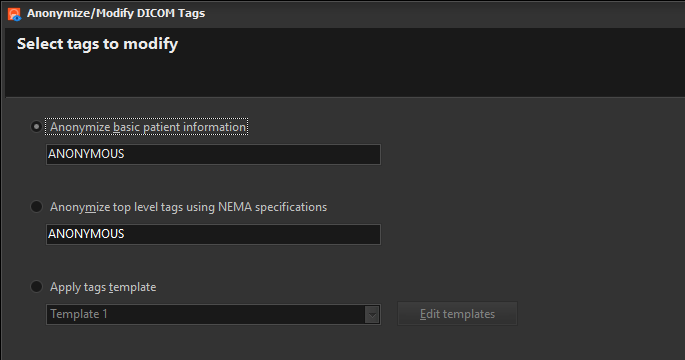
Anonymize basic patient information
This option anonymizes the most basic DICOM tags, which are the following:
- patient’s name (0010, 0010)
- patient ID (0010, 0020)
- patient birth date (0010, 0030)
- patient’s age (0010, 1010)
- patient’s sex (0010, 0040)
- comment (0010, 4000)
- institution name (0008, 0080)
- institution address (0008, 0081)
- institution department (0008, 1040)
- Issuer of Patient ID (0010, 0021)
- study description (0008, 1030)
- series description (0008, 103E)
- operator’s name (0008, 1070)
- referring physician’s name (0008, 0090)
- physician(s) of record (0008, 1048)
Anonymize top level tags using NEMA specification
This option anonymizes tags using the recommendations outlined in this link – http://dicom.nema.org/dicom/2013/output/chtml/part15/chapter_E.html#table_E.1-1.
Compared to the basic anonymization option above, this option anonymizes a lot more details.
Apply tags template
This option lets you apply a tags template to the selected images. A tags template is a set of changes you want to apply to the DICOM tags. You can create multiple templates for different purposes e.g. one template to modify certain tags to a specific value, another template to erase only patient details, and yet another tag to modify all patient, doctor and study details.
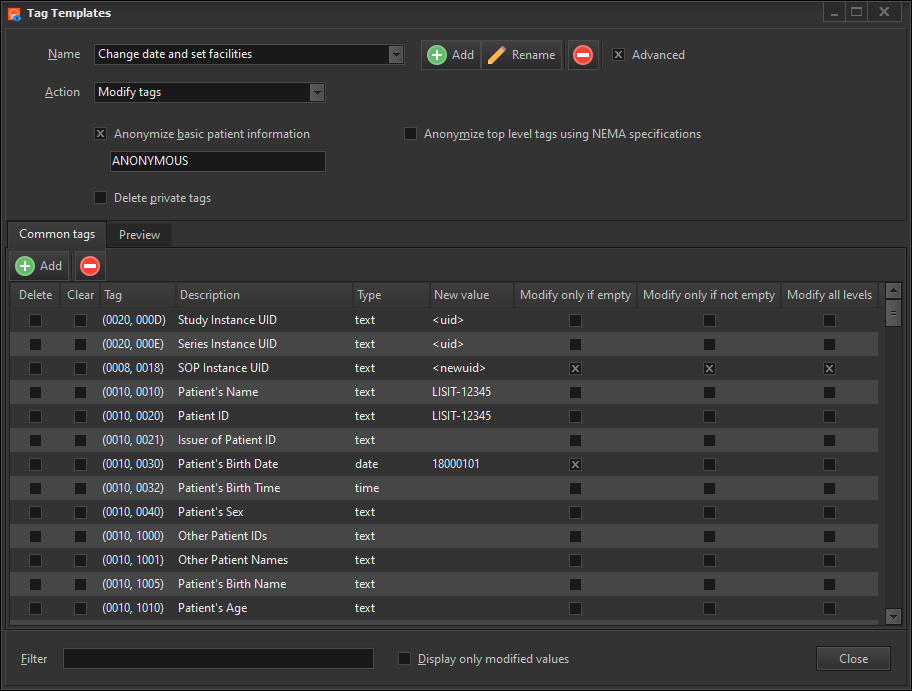
The Preview window will then show you how the tag values would appear in the modified images.
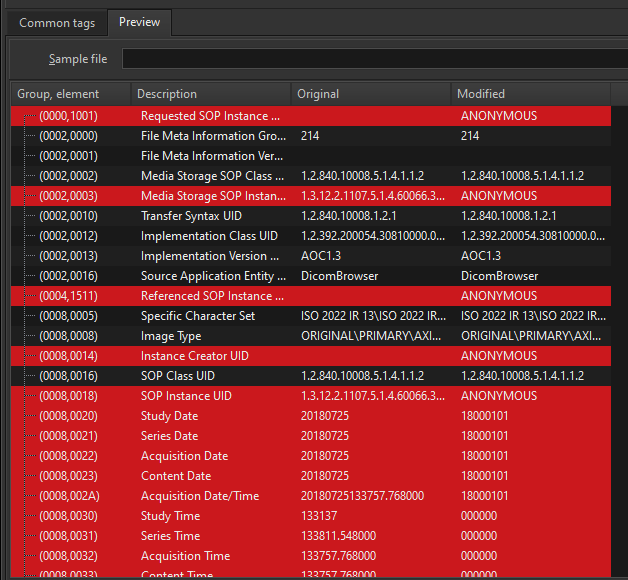
These 3 options give you complete flexibility as to how you want to anonymize the DICOM tags in your images.
Download a 14-day trial of Easy DICOM Viewer now, and see how you can easily identify which images require your attention.
Easy DICOM Viewer is a collaborative effort between LISIT, Co., Ltd. and Yohz Software. To learn more about Easy DICOM Viewer or download a trial, please use this link. If you are in Japan, please use this link instead.
See also: